HumaOrganoMatrix SCREEN
Human Organ ECM,
SKU: OSP
HumaOrganoMatrix SCREEN are tissue culture plates coated with native human-derived organ ECM solutions. HumaOrganoMatrix SCREEN is ideal for many applications including coating tissue culture surfaces to support cell attachment and growth.
Background
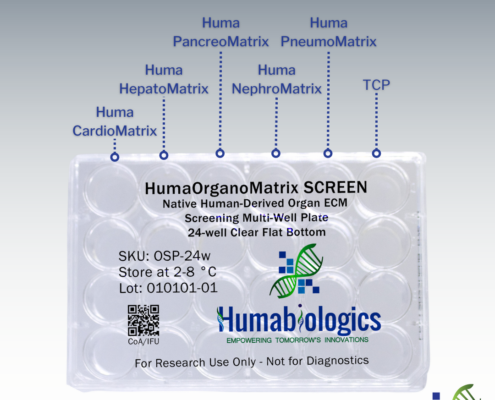
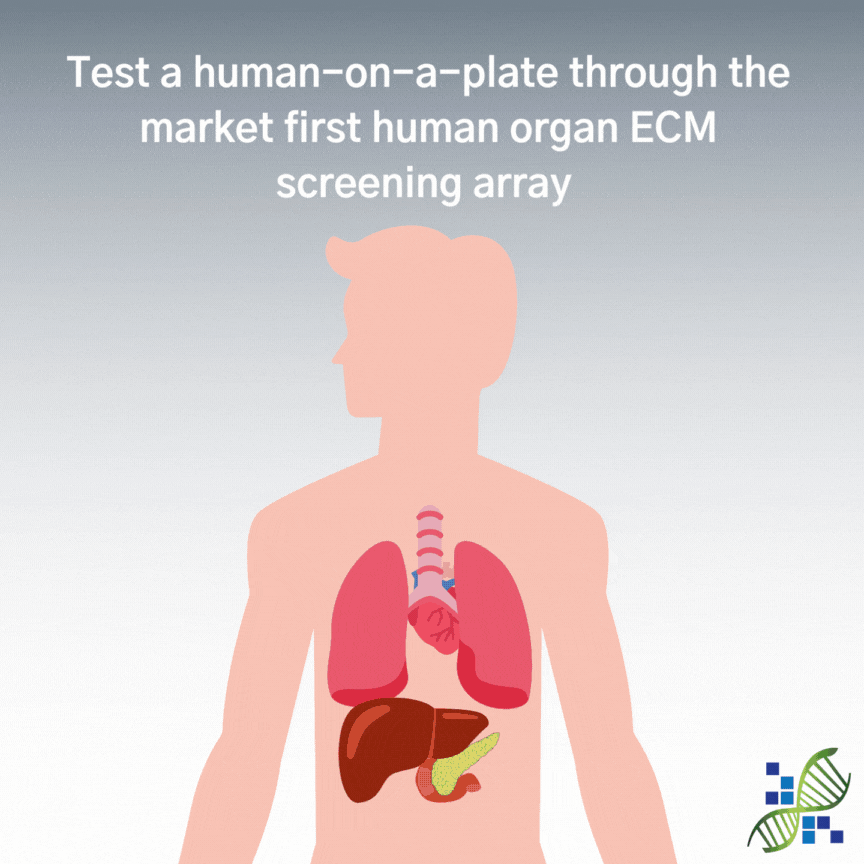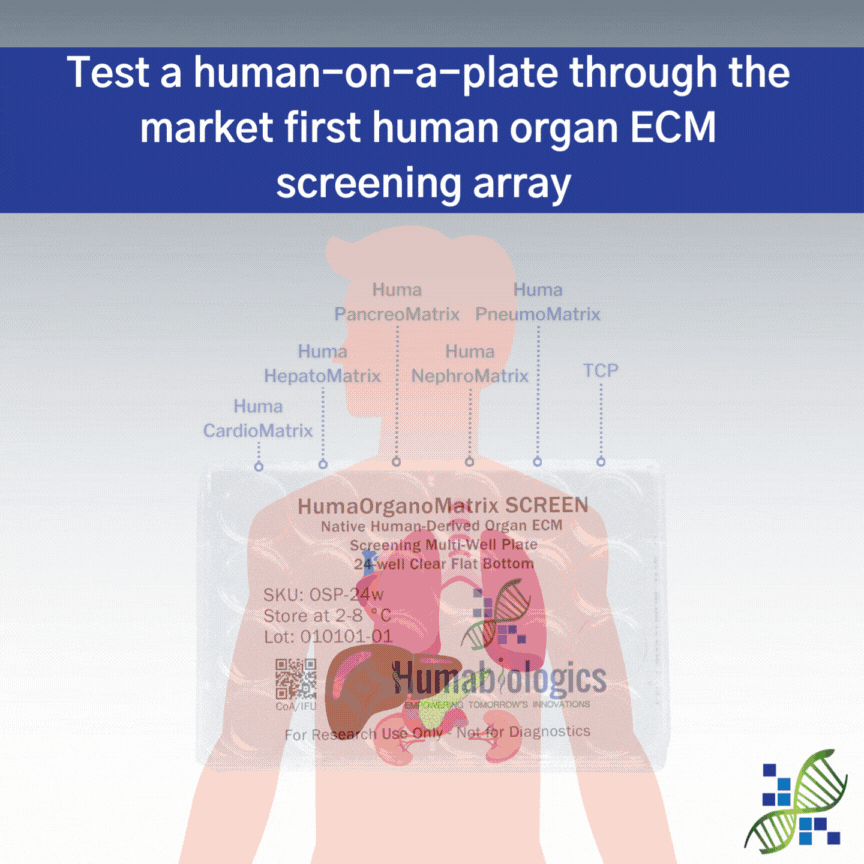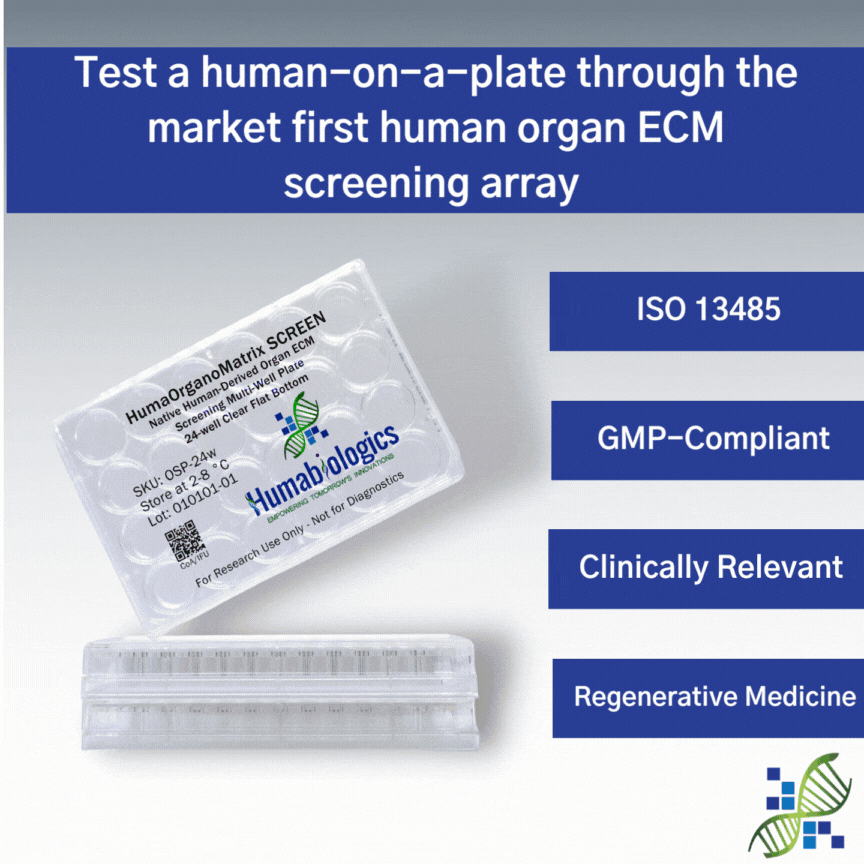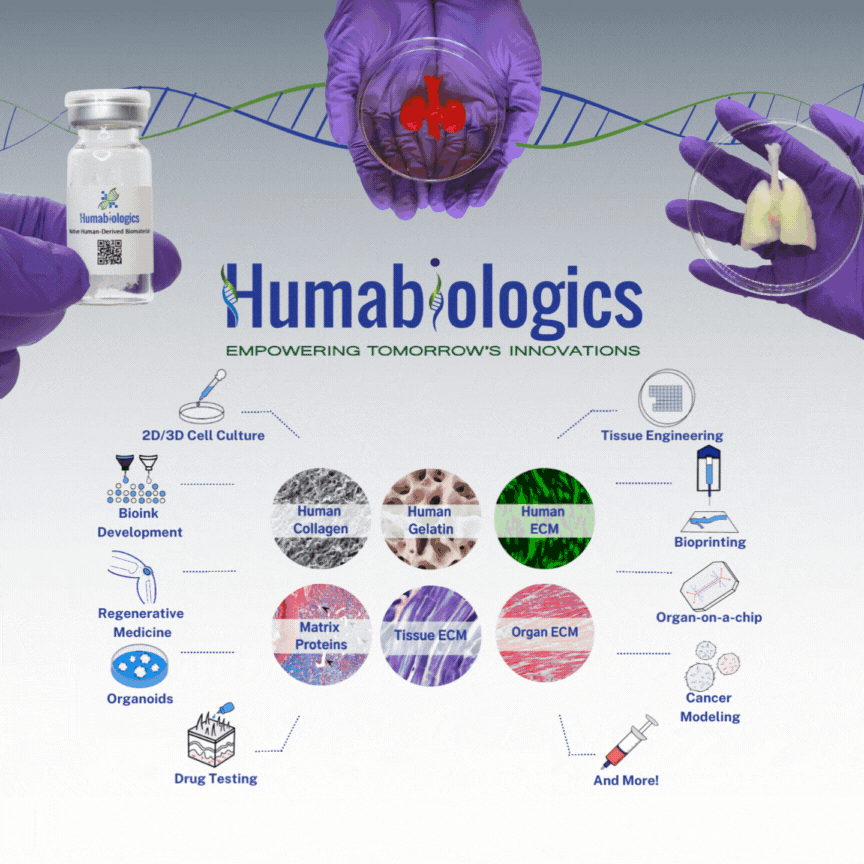
HumaOrganoMatrix SCREEN is a native human organ screening multi-well plate providing 6 test conditions. HumaOrganoMatrix SCREEN is coated with Huma CardioMatrix (Heart ECM), Huma PancreoMatrix (Pancreas ECM), Huma HepatoMatrix (Liver ECM), Huma NephroMatrix (Kidney ECM), and Huma PulmoMatrix (Lung ECM) to help determine which organ-specific ECM is best suited for your research needs. Each well is designed to deliver precise and independent testing conditions, ensuring the reliability of your data.
The organ-specific ECMs used in this product retain the native matrix proteins and growth factors found in their tissue, including collagens (Type I, III, IV, VI), glycoproteins (laminin, fibronectin, entactin, fibrillin, tenascin), and glycosaminoglycans (heparan sulfate, hyaluronic acid, chondroitin sulfate and dermatan sulfate).
HumaOrganoMatrix SCREEN reduces labor time, consumable costs, and accelerates therapy delivery without affecting the quality of cells.
Tissue Source
The ECMs used in HumaOrganoMatrix SCREEN are isolated from human tissue sourced strictly from American Association of Tissue Banks (AATB) accredited and FDA registered tissue banks and organ procurement organizations (OPOs). Humabiologics strives to meet research needs by providing high quality biomaterials obtained from tissue partners who comply with requirements for transplantable human tissues under 21 CFR 1271 of the FDA.
Precautions and Disclaimer
The ECMs used in HumaOrganoMatrix SCREEN are obtained from human tissue that has been tested and found negative for a minimum of HIV-1, HIV-2, hepatitis B, and hepatitis C, as well as other infectious agents. Please review the Safety Data Sheet (SDS) for information regarding hazards and safe handling practices. HumaOrganoMatrix SCREEN is for research use only and is not intended for human use, diagnosis, screening, household, food, or other uses.
Storage Conditions/Expiration
HumaOrganoMatrix SCREEN should be stored at 2 to 8 °C upon receipt. Do not freeze coated plates. Research grade products have a shelf life of 3 months from receipt.
Quality Testing
| Test | Specification |
|---|---|
| Cell Attachment | Pass |
| Protein Composition by SDS-PAGE and Coomassie Stain* | Pass |
| Immunology/Serology Testing | |
| HBV | Non-Reactive |
| HCV | Non-Reactive |
| HIV-1 | Non-Reactive |
| HIV-2 | Non-Reactive |
| RPR-Syphilis | Non-Reactive |
** Gel data is available upon request
** Testing was done using FDA approved kits in CLIA certified lab
*** Donor info is available upon request
Publications
Safety Data Sheet