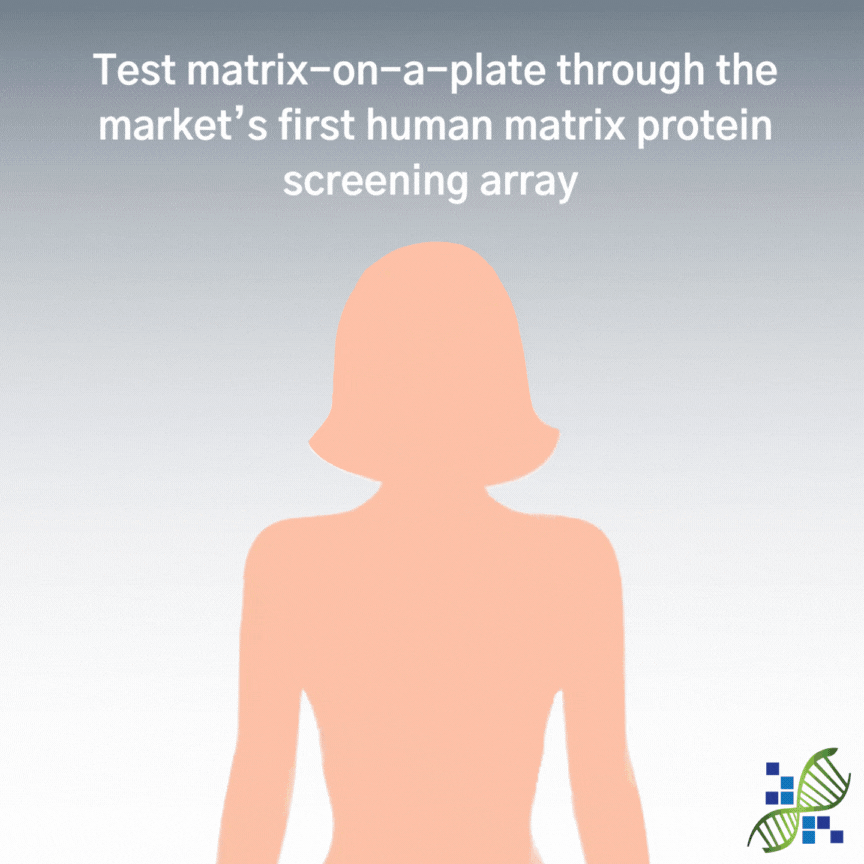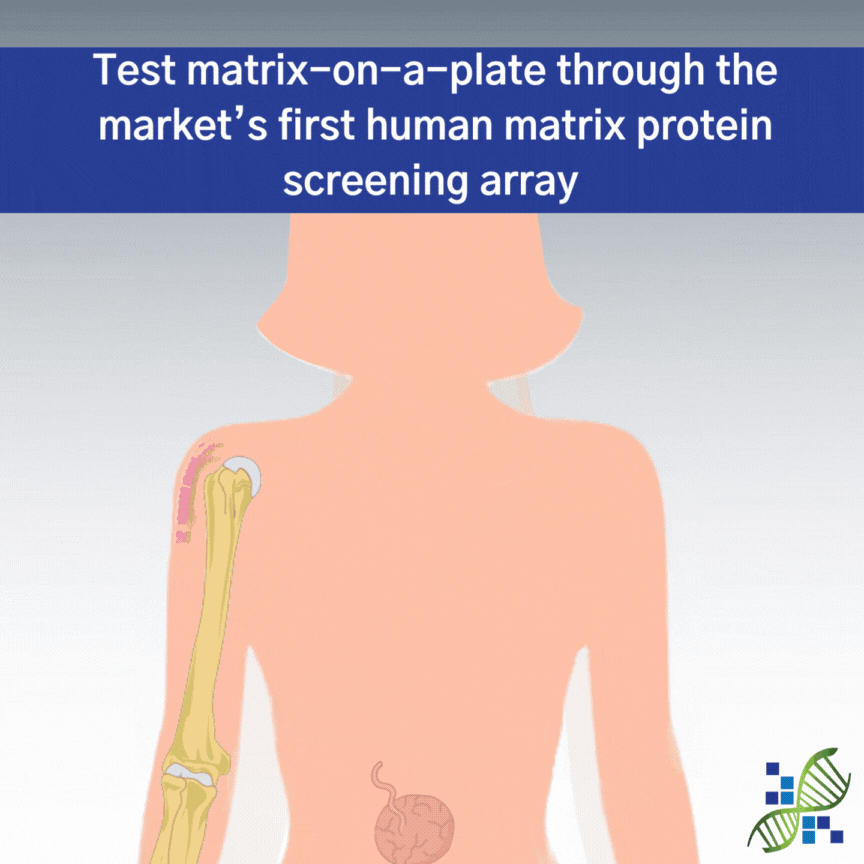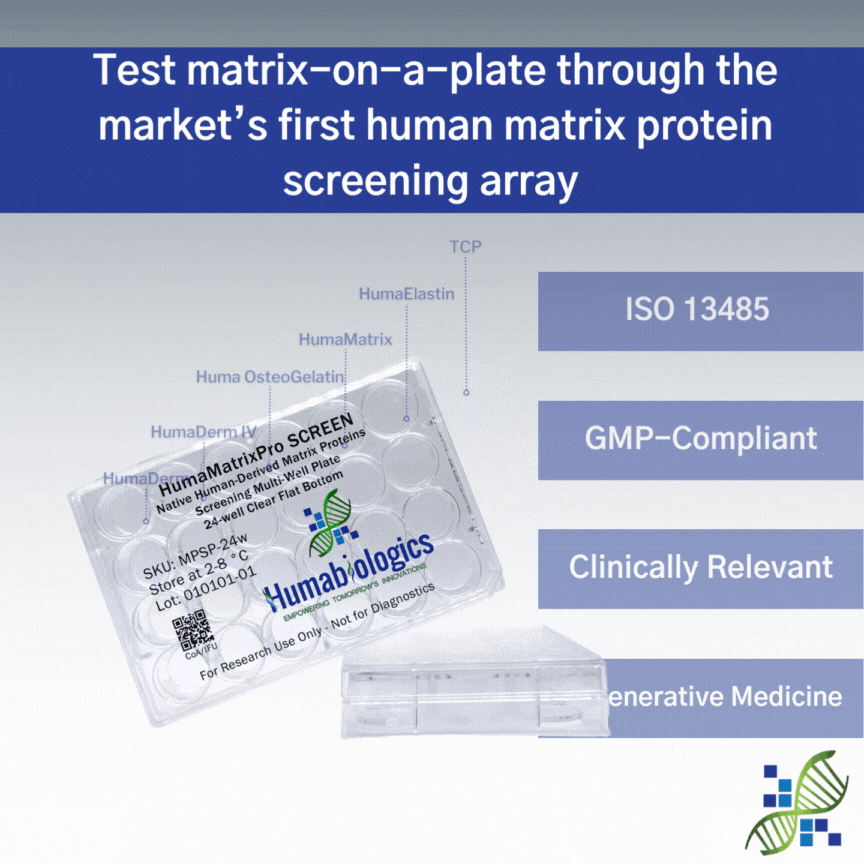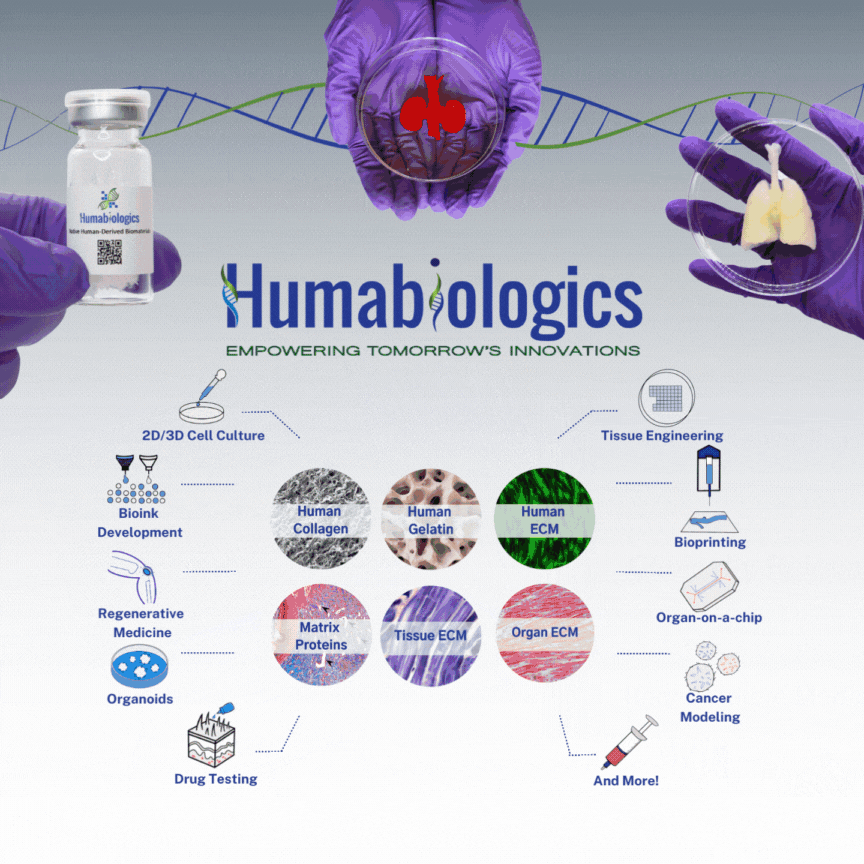
HumaMatrixPro SCREEN
Human Matrix Proteins
SKU: MPSP
HumaMatrixPro SCREEN are tissue culture plates coated with native human-derived collagen solutions. HumaMatrixPro SCREEN is ideal for many applications including coating tissue culture surfaces to support cell attachment and growth.
Background
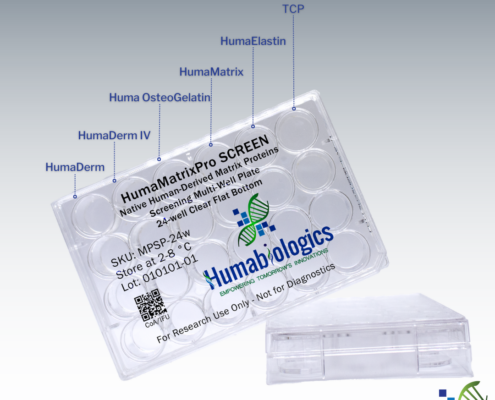
HumaMatrixPro SCREEN is a native human collagen screening multi-well plate providing 6 test conditions. HumaMatrixPro SCREEN is coated with HumaDerm (human skin collagen type I), HumaDerm IV (human collagen type IV), Huma OsteoGelatin (human bone gelatin), HumaElastin (human skin elastin), and HumaMatrix (universal human ECM) to help determine which matrix protein is best suited for your research needs. Each well is designed to deliver precise and independent testing conditions, ensuring the reliability of your data.
Type I Collagen is the most abundant type of collagen in the human body, as a major structural matrix protein in skin, and many other tissues (bone, tendon, and fibrous connective tissues). Type I collagen is a heterotrimer composed of one α2(I) chain and two α1(I) chains [1]. It is characterized by the presence of three regions on SDS-PAGE corresponding to molecular weights. HumaDerm, human skin collagen type I, presents all three regions and chains with very minimal fragmented proteins in between.
Type IV collagen occupies a distinct role within the realm of connective tissues. It is primarily associated with basement membranes, where it plays a pivotal role in mechanical stability and regulates critical functions including cell growth, differentiation, morphogenesis, homeostasis, and regeneration [2]. Type IV collagen can exist in at least three hetero-trimeric triple helical forms made up of three α chains [3,4]. HumaDerm IV, human collagen type IV, demonstrates the presence of intact α chains on SDS-PAGE.
Gelatin is a heterogenous mixture of water-soluble proteins with high average molecular masses. Gelatin proteins are derived by denaturing collagen-rich tissue in water [5]. Huma OsteoGelatin, human bone gelatin, supports rapid cell attachment and growth due to its excellent biocompatibility.
HumaMatrix, native human-derived ECM, is considered the universal human ECM which retains the native matrix proteins and growth factors found in human tissue including collagen, elastin, laminin, glycosaminoglycans and many other matrix proteins.
Elastin is a crucial structural protein found in various connective tissues throughout the body, providing elasticity and resilience to organs and tissues that require the ability to stretch and recoil [6]. It is primarily present in tissues such as the skin, blood vessels, lungs, and ligaments. Elastin is composed of amino acids – predominantly glycine, valine, alanine, and proline, with unique cross-linking patterns that contribute to its distinctive properties [7].
HumaMatrixPro SCREEN reduces labor time, consumable costs, and accelerates therapy delivery without affecting the quality of cells.
Tissue Source
The matrix proteins used in HumaMatrixPro SCREEN are isolated from human tissue sourced strictly from American Association of Tissue Banks (AATB) accredited and FDA registered tissue banks and organ procurement organizations (OPOs). Humabiologics strives to meet research needs by providing high quality biomaterials obtained from tissue partners who comply with requirements for transplantable human tissues under 21 CFR 1271 of the FDA.
Precautions and Disclaimer
The matrix proteins used in HumaMatrixPro SCREEN are obtained from human tissue that has been tested and found negative for a minimum of HIV-1, HIV-2, hepatitis B, and hepatitis C, as well as other infectious agents. Please review the Safety Data Sheet (SDS) for information regarding hazards and safe handling practices. HumaMatrixPro SCREEN is for research use only and is not intended for human use, diagnosis, screening, household, food, or other uses.
Storage Conditions/Expiration
HumaMatrixPro SCREEN should be stored at 2 to 8 °C upon receipt. Do not freeze coated plates. Research grade products have a shelf life of 3 months from receipt.
Quality Testing
| Test | Specification |
|---|---|
| Cell Attachment | Pass |
| Purity/Identity/Protein Composition by SDS-PAGE and Coomassie Stain* | Pass |
| Immunology/Serology Testing | |
| HBV | Non-Reactive |
| HCV | Non-Reactive |
| HIV-1 | Non-Reactive |
| HIV-2 | Non-Reactive |
| RPR-Syphilis | Non-Reactive |
** Gel data is available upon request
** Testing was done using FDA approved kits in CLIA certified lab
*** Donor info is available upon request
Publications
Safety Data Sheet